Health and Medicine

Research
Disparities in sleep health and insomnia may begin at a young age
Black children were 2.6 times more likely to experience insomnia that begins in childhood and persists through young adulthood compared to white children
April 05, 2024
Latest News
Health and Medicine News RSS Feed
Q&A: Can weight loss drugs help in addiction treatment?
April 11, 2024

New role for bacterial enzyme in gut metabolism revealed
February 28, 2024

Improving efficiency, reliability of AI medical summarization tools
February 21, 2024

Heard on campus: Nikki Crowley on 30 years of neuroscience advances
February 13, 2024
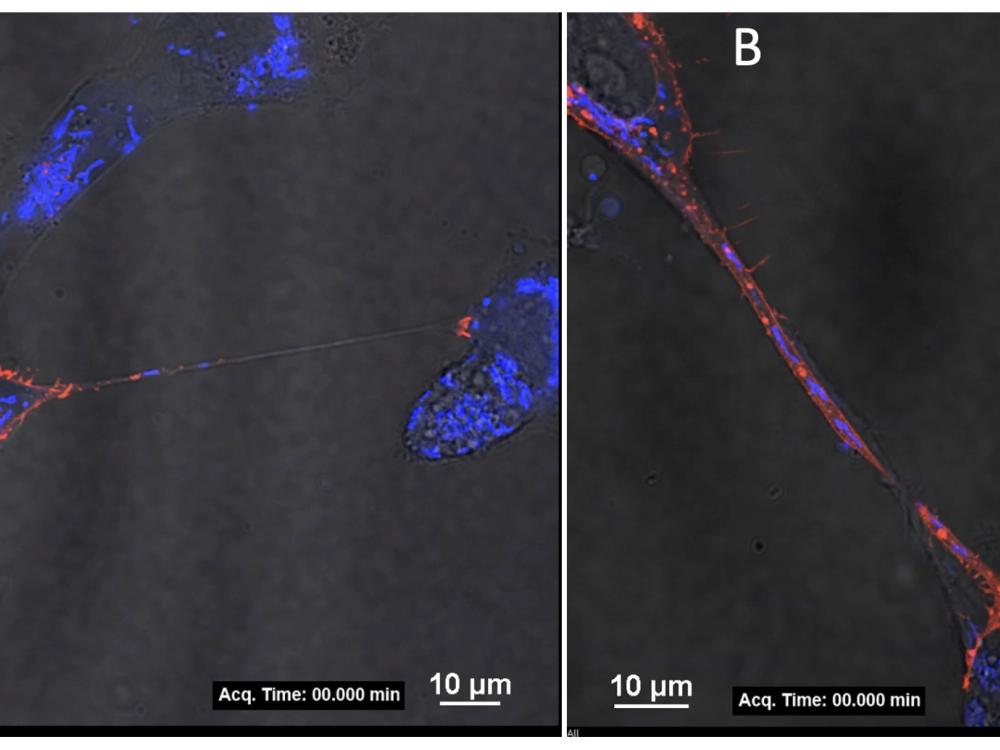
How does Zika virus replicate and transmit from mother to fetus?
February 05, 2024

Undergraduate research may contribute to COVID-19 treatments
January 25, 2024
Foodborne-pathogen Listeria may hide from sanitizers in biofilms
January 24, 2024

Interactive screen use reduces sleep time in kids, researchers find
December 13, 2023

Bacteria's mucus maneuvers: Study reveals how snot facilitates infection
December 05, 2023

The golden rule may get a boost when teens feel connected to others
November 28, 2023

Study of sourdough starter microbiomes to boost bread quality and safety
November 21, 2023

Q&A: Modeling measles amidst a global disruption in vaccine supplies
November 16, 2023

Q&A: What can we do to prevent and control hypertension?
November 15, 2023

Understanding the barriers to taxing alcohol and tobacco in Nepal
November 13, 2023

Q&A: Can virtual reality help people eat a healthier diet?
November 10, 2023

NIH diversity grant to fund student’s 3D bioprinting research
November 08, 2023
Latest News
Health and Medicine News RSS Feed






Q&A: Can weight loss drugs help in addiction treatment?
April 11, 2024















New role for bacterial enzyme in gut metabolism revealed
February 28, 2024

Improving efficiency, reliability of AI medical summarization tools
February 21, 2024


Heard on campus: Nikki Crowley on 30 years of neuroscience advances
February 13, 2024



How does Zika virus replicate and transmit from mother to fetus?
February 05, 2024





Undergraduate research may contribute to COVID-19 treatments
January 25, 2024


Foodborne-pathogen Listeria may hide from sanitizers in biofilms
January 24, 2024






Interactive screen use reduces sleep time in kids, researchers find
December 13, 2023


Bacteria's mucus maneuvers: Study reveals how snot facilitates infection
December 05, 2023




The golden rule may get a boost when teens feel connected to others
November 28, 2023


Study of sourdough starter microbiomes to boost bread quality and safety
November 21, 2023

Q&A: Modeling measles amidst a global disruption in vaccine supplies
November 16, 2023



Q&A: What can we do to prevent and control hypertension?
November 15, 2023

Understanding the barriers to taxing alcohol and tobacco in Nepal
November 13, 2023

Q&A: Can virtual reality help people eat a healthier diet?
November 10, 2023


NIH diversity grant to fund student’s 3D bioprinting research
November 08, 2023

Get the news by email
Subscribe